Multiple Choice
Consider the following thermochemical equation: CaO(s) + H2O( 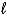 ) Ca(OH) 2(s) H = -66.5 kJ Which of the following statements is/are correct?
) Ca(OH) 2(s) H = -66.5 kJ Which of the following statements is/are correct?
(i) The reaction is exothermic
(ii) The reaction releases 66.5 kJ per gram of Ca(OH) 2(s) formed
(iii) Alternatively,the H term could be added to the product side of the equation
A) i only
B) ii only
C) i and ii
D) i and iii
E) i,ii,and iii
Correct Answer:

Verified
Correct Answer:
Verified
Q1: In the complete combustion of C<sub>3</sub>H<sub>8</sub>O<sub>3</sub>,how many
Q11: Aqueous HCl is added to an
Q13: When one mole of gaseous hydrogen
Q18: How many moles of oxygen are consumed
Q19: The reaction 2 Sb + 3
Q20: In the combustion of natural gas
Q21: In reacting aluminum carbonate with hydrochloric
Q32: A chemical reaction resulted in 1.376 g
Q37: What is the theoretical yield of phosphorus
Q40: Which of the following best describes a