Multiple Choice
Consider the reaction HC2H3O2(aq) + H2O(l) H3O+(aq) + C2H3O2-(aq) . Which species is the conjugate acid?
A) HC2H3O2(aq)
B) H2O(l)
C) H3O+(aq)
D) 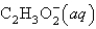
E) two of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: A solution has [H<sup>+</sup>] = 5.0 *10<sup>-</sup><sup>8</sup>
Q14: A buffered solution contains HNO<sub>2</sub>. It also
Q15: A solution has [H<sup>+</sup>] = 4.3 *10<sup>-</sup><sup>8</sup>
Q16: Which statement is true for a strong
Q17: Solid calcium hydroxide is dissolved in water
Q19: What is the pH of a solution
Q20: Calculate the [H<sup>+</sup>] in a solution that
Q21: A solution has [OH<sup>-</sup>]= 5.0 *10<sup>-</sup><sup>4</sup> M.
Q22: Calculate the pH of 0.056 M HClO<sub>4</sub>.<br>A)
Q23: The pH of a solution at 25<sup>o</sup>C