Multiple Choice
Calculate the quantity of energy required to change 22.8 g of liquid water to steam at 100oC. The molar heat of vaporization of water is 40.6 kJ/mol.
A) 10.1 kJ
B) 51.4 kJ
C) 0.562 kJ
D) 32.1 kJ
E) 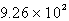 kJ
kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: What is the major attractive force in
Q27: Select the two compounds that are more
Q28: Which of the following is true for
Q29: The normal boiling point of water is<br>A)
Q30: The freezing point of helium is approximately
Q32: The normal boiling point of liquid W
Q33: Select the two compounds that are more
Q34: The specific heat capacity of liquid water
Q35: Of the following substances, choose the one
Q36: When a water molecule forms a hydrogen