Multiple Choice
The molar heat of fusion of water is 6.02 kJ/mol. Calculate the energy required to melt 44.3 g of water.
A) 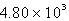 kJ
kJ
B) 267 kJ
C) 2.46 kJ
D) 133 kJ
E) 14.8 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: Name the type of crystalline solid formed
Q16: What is the major attractive force in
Q17: The normal freezing point of water is<br>A)
Q18: As the temperature of a liquid increases,
Q19: Calculate the quantity of energy required to
Q21: Consider the following compounds: CO NH<sub>3</sub> CO<sub>2</sub>
Q22: At 1 atm of pressure and a
Q23: Which of the following has the lowest
Q24: Consider the following compounds: CO NH<sub>3</sub> CO<sub>2</sub>
Q25: Which of the following should have the