Multiple Choice
The density of copper is 8.92 g/mL. The mass of a piece of copper that has a volume of 10.4 mL is
A) 0.928 g
B) 928 g
C) 92.8 g
D) 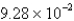 g
g
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q72: Express 1840000 in scientific notation.<br>A) 5.41 *
Q73: Convert 316718.0 mm to kilometers.<br>A) 3.167180 km<br>B)
Q74: In the sum of 54.34 + 45.66,
Q75: How many significant figures are in the
Q76: Which of the following is an SI
Q78: Convert 9.75 kg to pounds (1 lb
Q79: The number 600,000 expressed in scientific notation
Q80: How many significant figures are in the
Q81: Perform the following conversion: 5.39 m/s =
Q82: The number of milligrams in 6.6 kg