Multiple Choice
The density of gold is 19.3 g/mL. What is the volume of a gold nugget that weighs 68.7 g?
A) 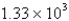 mL
mL
B) 3.56 mL
C) 0.281 mL
D) 49.4 mL
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: How many significant figures are in the
Q13: Convert: 24.0 cc = _ mL.<br>A) 240
Q14: Convert: -47.9°C = _ °F.<br>A) -118.2°F<br>B) 5.4°F<br>C)
Q15: Convert 949.0 L to milliliters.<br>A) 0.9490 mL<br>B)
Q16: What is the Celsius equivalent of 436.5
Q18: Convert 643.5 mi to kilometers (1 m
Q19: An object is 153.8 inches in height.
Q20: How many significant figures are in the
Q21: Using the rules of significant figures, calculate
Q22: Using the rules of significant figures, calculate