Multiple Choice
The pressure of an ideal gas is doubled in an isothermal process.The root-mean-square speed of the molecules:
A) does not change
B) increases by a factor of 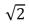
C) decreases by a factor of 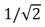
D) increases by a factor of 2
E) decreases by a factor of 1/2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: Over 1 cycle of a cyclic process
Q27: An ideal gas undergoes an isothermal process
Q28: Assume that helium behaves as an ideal
Q29: In order that a single process be
Q30: Energy transferred into an ideal gas as
Q32: An adiabatic process for an ideal gas
Q33: The "Principle of equipartition of energy" states
Q34: The temperature of low pressure hydrogen is
Q35: Assume that helium behaves as an ideal
Q36: The mean free path of molecules in