Multiple Choice
A sample of argon gas (molar mass 40 g) is at four times the absolute temperature of a sample of hydrogen gas (molar mass 2 g) .The ratio of the rms speed of the argon molecules to that of the hydrogen is:
A) 1
B) 5
C) 1/5
D) 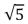
E) 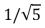
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q43: Evidence that molecules of a gas are
Q44: How many molecules are in a sample
Q45: According to the Maxwellian speed distribution, as
Q46: A monatomic gas can have internal energy
Q47: The temperature of a gas is most
Q49: Two identical rooms in a house are
Q50: As the pressure in an ideal gas
Q51: A real gas is changed slowly from
Q52: The specific heat at constant volume of
Q53: The ratio of the specific heat of