Multiple Choice
The diagram shows three isotherms for an ideal gas, with T3-T2 the same as T2-T1.It also shows five thermodynamic processes carried out on the gas.Rank the processes in order of the change in the internal energy of the gas, least to greatest. 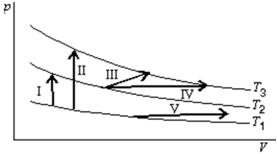
A) I, II, III, IV, V
B) V; then I, III and IV tied; then II
C) V; I; then III, and IV tied; then II
D) II; then I, III and IV tied; then V
E) II; I; then III, IV, and V tied
Correct Answer:

Verified
Correct Answer:
Verified
Q76: If a gas expands freely into a
Q77: The mean free path of molecules in
Q78: The mean free path of air molecules
Q79: According to the Maxwellian speed distribution, as
Q80: A quantity of an ideal gas is
Q82: Two ideal monatomic gases are in thermal
Q83: The specific heat of a polyatomic gas
Q84: The energy absorbed as heat by an
Q85: An isothermal process for an ideal gas
Q86: For a given change in temperature, the