Multiple Choice
An ideal gas of N diatomic molecules has temperature T.If the number of molecules is doubled without changing the temperature, the internal energy increases by:
A) 0
B) 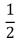 NkT
NkT
C) 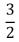 NkT
NkT
D) 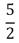 NkT
NkT
E) 3 NkT
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: An ideal diatomic gas has a molar
Q13: Air is pumped into a bicycle tire
Q14: Five molecules have speeds of 2.8, 3.2,
Q15: An ideal monatomic gas has a molar
Q16: The average speed of air molecules at
Q18: An ideal gas of N monatomic molecules
Q19: A given mass of gas is enclosed
Q20: The force on the walls of a
Q21: The temperature of n moles of
Q22: The specific heat C<sub>v</sub> at constant volume