Multiple Choice
During a reversible adiabatic expansion of an ideal gas, which of the following is NOT true?
A) 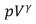 = constant
= constant
B) pV = nRT
C) 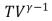 = constant
= constant
D) W = - V pdV
E) pV = constant
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q89: The heat capacity at constant volume and
Q90: The pressure of an ideal gas of
Q91: During a slow adiabatic expansion of a
Q92: The pressure of an ideal gas is
Q93: As the volume of an ideal gas
Q95: Ideal monatomic gas A is composed of
Q96: A real gas undergoes a process which
Q97: Oxygen (molar mass = 32 g)occupies a
Q98: If the temperature T of an ideal
Q99: In using the ideal gas law, the