Multiple Choice
An ideal gas is to taken reversibly from state i, at temperature T1, to other states labeled I, II, III, IV and V on the p-V diagram below.All are at the same temperature T2. Rank the five processes according to the change in entropy of the gas, least to greatest. 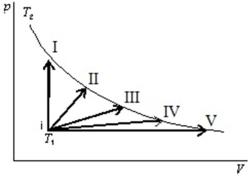
A) I, II, III, IV, V
B) V, IV, III, II, I
C) I, then II, III, IV, and V tied
D) I, II, III, and IV, tied, then V
E) I and V tied, then II, III, IV
Correct Answer:

Verified
Correct Answer:
Verified
Q51: One mole of an ideal gas expands
Q52: A force of 5 N stretches an
Q53: A Carnot cycle heat engine operates between
Q54: A heat engine that in each cycle
Q55: An inventor suggests that a house might
Q56: A Carnot refrigerator runs between a
Q58: According to the second law of thermodynamics:<br>A)all
Q59: For all irreversible processes involving a system
Q60: For one complete cycle of a reversible
Q61: A cyclical process that transfers energy as