Multiple Choice
A refrigerator absorbs energy of magnitude 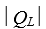 as heat from a low temperature reservoir and rejects energy of magnitude
as heat from a low temperature reservoir and rejects energy of magnitude 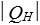 as heat to a high temperature reservoir.Work W is done on the working substance.The coefficient of performance is given by:
as heat to a high temperature reservoir.Work W is done on the working substance.The coefficient of performance is given by:
A) 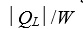
B) 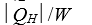
C) 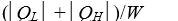
D) 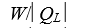
E) 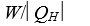
Correct Answer:

Verified
Correct Answer:
Verified
Q6: On a warm day a pool of
Q7: A slow (quasi-static)process is NOT reversible if:<br>A)the
Q8: Consider the following processes: The temperatures of
Q9: Let k be the Boltzmann constant.If the
Q10: The temperature T<sub>L</sub> of the cold reservoirs
Q12: A Carnot heat engine operates between a
Q13: The change in entropy is zero for:<br>A)reversible
Q14: The difference in entropy <span
Q15: A Carnot heat engine runs between
Q16: Possible units of entropy are:<br>A)J<br>B)J/K<br>C)J<sup>-1</sup><br>D)liter.atm<br>E)cal/mol