Multiple Choice
The figure shows the energy levels for an electron in a finite potential energy well.If the electron makes a transition from the n = 3 state to the ground state, what is the wavelength of the emitted photon? 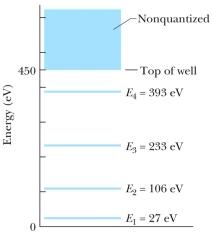
A) 6.0 nm
B) 5.7 nm
C) 5.3 nm
D) 3.0 nm
E) 2.3 nm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: The quantum number n is most closely
Q28: A particle is trapped in a finite
Q29: The radial probability density for the electron
Q30: A particle is trapped in an infinite
Q31: The Balmer series of hydrogen is important
Q33: An electron confined in a one-dimensional infinite
Q34: Which of the following sets of quantum
Q35: Take the potential energy of a hydrogen
Q36: A particle in a certain finite potential
Q37: The wave function for an electron in