Multiple Choice
The figure shows the energy levels for an electron in a finite potential energy well.If an electron in the n = 2 state absorbs a photon of wavelength 2.0 nm, what happens to the electron? 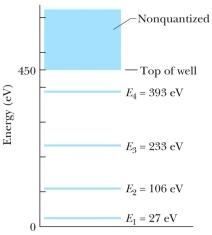
A) It makes a transition to the n = 3 state.
B) It makes a transition to the n = 4 state.
C) It escapes the well with a kinetic energy of 280 eV.
D) It escapes the well with a kinetic energy of 730 eV.
E) Nothing; this photon does not have an energy corresponding to an allowed transition so it is not absorbed.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: A particle is trapped in an infinite
Q2: Consider the following: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6585/.jpg" alt="Consider the
Q3: A particle is trapped in a one-dimensional
Q5: An electron is in a one-dimensional trap
Q6: If the wave function <img
Q7: A particle is confined to a one-dimensional
Q8: If a wave function <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6585/.jpg"
Q9: If P(r)is the radial probability density for
Q10: The following image is a dot plot
Q11: Four different particles are trapped in one-dimensional