Multiple Choice
The magnitude of the orbital magnetic dipole moment 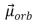 of an atom is ( B is the Bohr magneton, and
of an atom is ( B is the Bohr magneton, and 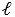 is a positive integer) :
is a positive integer) :
A) " B"
B) " B11ef01a9_ebb2_0dc7_bc85_ad2d297ddd86_TB6585_11"
C) " B
11eab079_97cd_a77a_a51f_8d1202e622a8_TB6585_11"
D) " B (211ef01a9_ebb2_0dc7_bc85_ad2d297ddd86_TB6585_11+1) "
E) " B 11ef01a9_ebb2_0dc7_bc85_ad2d297ddd86_TB6585_112"
Correct Answer:

Verified
Correct Answer:
Verified
Q62: The Einstein-de Haas experiment showed that:<br>A)atoms emit
Q63: The K <sub> <span class="ql-formula" data-value="\infty"><span
Q64: A laser must be pumped to achieve:<br>A)a
Q65: The quantity L<sub>z</sub> is related to the
Q66: For any atom other that hydrogen and
Q68: Space quantization means that:<br>A)space is quantized<br>B)L<sub>z</sub> can
Q69: If electrons did not have intrinsic angular
Q70: Photons in a laser beam have the
Q71: An electron in an atom is in
Q72: Six electrons are in a two-dimensional square