Multiple Choice
According to the titration curve to the right, acid A is _____ because the pH _____. 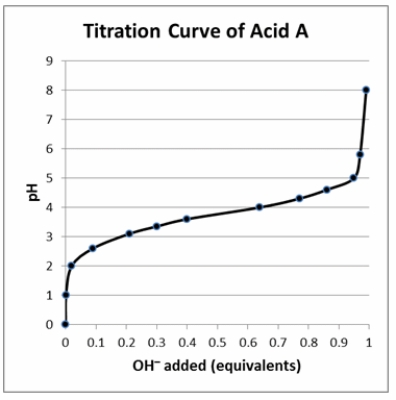
A) weak; resists change when 50% titrated.
B) strong; resists change when 50% titrated.
C) weak; changes dramatically when 100% titrated.
D) strong; changes dramatically when 100% titrated.
E) It cannot be determined from the information given.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Ice is _ than water because _.<br>A)
Q2: Which statement about buffers is TRUE?<br>A) A
Q3: Briefly define "isotonic," "hypotonic," and "hypertonic" solutions.
Q5: A 1.0 M solution of a compound
Q6: Which of the following would have the
Q7: Which property of water has had the
Q8: The high degree of cohesion of water
Q9: Which statement does NOT describe a reason
Q10: Which statement does NOT describe a strategy
Q11: Explain the fact that triethylammonium chloride ((CH<sub>3</sub>CH<sub>2</sub>)<sub>3</sub>N-HCl)