Multiple Choice
Polar molecules cannot easily pass through the cell membrane, but hydrophobic molecules can easily pass through the membrane. The two molecules shown in the diagram both have effects that include raising blood pressure. Comparing two molecules to the right, which statement is TRUE? 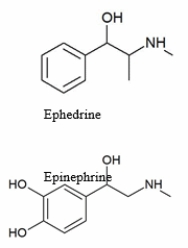
A) Ephedrine can more easily pass through the cell membrane than epinephrine.
B) Epinephrine can more easily pass through the cell membrane than ephedrine.
C) Both epinephrine and ephedrine can pass through the cell membrane equally well.
D) Neither epinephrine nor ephedrine can pass through the cell membrane.
E) None of the statements is true.
Correct Answer:

Verified
Correct Answer:
Verified
Q37: Speculate why weak forces, not strong forces,
Q38: Camels live in very dry habitats and
Q39: A compound is known to have a
Q40: Dissolved solutes alter some physical (colligative) properties
Q41: If the K<sub>a</sub> of an acid is
Q43: According to the titration curve above, acid
Q44: The aqueous solution with the HIGHEST pH
Q45: Formic acid is used in the venom
Q46: Which compound acts as a diprotic acid?<br>A)
Q47: In proteins, the amino acid histidine (His)