Glycerol 3-Phosphate Dehydrogenase Catalyzes the Following Reversible Reaction:
Glycerol 3-Phosphate
Essay
Glycerol 3-phosphate dehydrogenase catalyzes the following reversible reaction:
glycerol 3-phosphate + NAD+ NADH + H+ + dihydroxyacetone phosphate
Given the standard reduction potentials below, calculate G'° for the glycerol 3-phosphate dehydrogenase reaction, proceeding from left to right as shown. Show your work. (The Faraday constant, 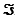 , is 96.48 kJ/V · mol.)
, is 96.48 kJ/V · mol.)
dihydroxyacetone phosphate + 2e- + 2H+ glycerol 3-phosphate
E'° = -0.29 V
NAD+ + H+ + 2e- NADH
E'° = -0.32 V
Correct Answer:

Answered by ExamLex AI
To calculate the standard Gibbs free ene...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Answered by ExamLex AI
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q2: Which redox reaction(s) will NOT proceed
Q3: When a mixture of glucose 6-phosphate
Q4: Which statement is TRUE regarding coupled reactions?<br>A)
Q5: Which statement about NAD<sup>+</sup> and NADH
Q6: Which molecule is NOT considered a universal
Q8: Which statement regarding redox reactions is TRUE?<br>A)
Q9: In general, when ATP hydrolysis is coupled
Q10: Explain why each of the following
Q11: Which statement is NOT true regarding the
Q12: Which compound has the LARGEST negative