Lactate Dehydrogenase Catalyzes the Reversible Reaction:
Pyruvate + NADH Lactate + NAD+
Given the Following Facts (A) Tell in H+
Short Answer
Lactate dehydrogenase catalyzes the reversible reaction:
pyruvate + NADH + H+ lactate + NAD+
Given the following facts (a) tell in which direction the reaction will tend to go if NAD+, NADH, pyruvate, and lactate were mixed, all at 1 M concentrations, in the presence of lactate dehydrogenase at pH 7; (b) calculate G'° for this reaction. Show your work.
NAD+ + H+ + 2e- NADH
E'° = -0.32 V
pyruvate + 2H+ + 2e- lactate
E'° = -0.19 V
The Faraday constant, 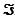 , is 96.48 kJ/V · mol.
, is 96.48 kJ/V · mol.
Correct Answer:

Answered by ExamLex AI
To answer the question, we need to consi...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Answered by ExamLex AI
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q65: Under standard conditions, is the oxidation of
Q66: <span class="ql-formula" data-value="\Delta"><span class="katex"><span class="katex-mathml"><math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow><mi mathvariant="normal">Δ</mi></mrow><annotation
Q67: The free energy of hydrolysis of
Q68: Which statement is TRUE with respect
Q69: glucose 6-phosphate <span class="ql-formula" data-value="\rightarrow"><span
Q71: Classify each of the *ed atoms as
Q72: Which atom is the LEAST electronegative?<br>A) C<br>B)
Q73: Which statement is NOT true for the
Q74: _ is used to illustrate the
Q75: Acyl phosphates, phosphoesters, and phosphaoanhydrides are