Essay
Alcohol dehydrogenase catalyzes the following reversible reaction:
acetaldehyde + NADH + H+ ethanol + NAD+
Use the following information to answer the questions below:
acetaldehyde + 2H+ + 2e- ethanol
E'° = -0.20 V
NAD+ + H+ + 2e- NADH
E'° = -0.32 V
The Faraday constant, 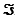 , is 96.48 kJ/V · mol.
, is 96.48 kJ/V · mol.
(a) Calculate G'° for the reaction as written. Show your work.
(b) Given your answer to (a), what is the G'° for the reaction occurring in the reverse direction?
(c) Which reaction (forward or reverse) will tend to occur spontaneously under standard conditions?
(d) In the cell, the reaction actually proceeds in the direction that has a positive G'°. Explain how this could be possible.
Correct Answer:

Answered by ExamLex AI
(a) To calculate the standard Gibbs free...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Answered by ExamLex AI
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q29: The structure of NAD<sup>+</sup> does NOT include:<br>A)
Q30: In glycolysis, the enzyme pyruvate kinase
Q31: For the reaction A <span
Q32: Under cellular conditions (37°C), what is
Q33: Which statement is TRUE for the
Q35: Given that the <span class="ql-formula"
Q36: In the following reaction, which molecule
Q37: Which statement is NOT true?<br>A) The
Q38: Which atom is a possible nucleophile?<br>A) an
Q39: Which biochemical reaction is NOT used in