Multiple Choice
What is the volume of carbon dioxide at STP from the decomposition of 2.50 g of potassium hydrogen carbonate (100.12 g/mol) ? __KHCO₃(s) 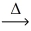 __k₂CO₃(s) + __H₂O(l) + __CO₂(g)
__k₂CO₃(s) + __H₂O(l) + __CO₂(g)
A) 0.280 L
B) 0.559 L
C) 1.12 L
D) 5.60 L
E) 22.3 L
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q28: Considering the limiting reactant,what is the volume
Q29: How many moles of oxygen gas react
Q30: Which of the following gases is blown
Q31: What term expresses the ratio of moles
Q32: What is the mass of insoluble lead(II)iodide
Q34: What principle states the volumes of gases
Q35: Which of the following is used to
Q36: What is the mass of hydrogen gas
Q37: How many moles of oxygen gas react
Q38: How many moles of hydrogen iodide are