Multiple Choice
What is the volume of carbon dioxide at STP from the decomposition of 5.00 g of bismuth carbonate (597.99 g/mol) ? __BI₂(CO₃) 3(s) 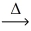 __BI₂O₃(s) + __CO₂(g)
__BI₂O₃(s) + __CO₂(g)
A) 0.0445 L
B) 0.0624 L
C) 0.187 L
D) 0.400 L
E) 0.562 L
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: How was Haber able to combine nitrogen
Q5: What principle states that mass is neither
Q6: What term refers to a type of
Q7: Considering the limiting reactant concept,how many moles
Q8: Considering the limiting reactant,what is the mass
Q10: How many moles of hydrogen peroxide decompose
Q11: What is the volume of hydrogen gas
Q12: Which two trace elements are usually found
Q13: How many moles of carbon monoxide react
Q14: Which of the following important industrial chemicals