Multiple Choice
Considering the limiting reactant,what is the mass of zinc sulfide (97.46 g/mol) produced from 0.750 g of zinc and 0.750 g of sulfur? Zn(s) + S(s) 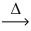 ZnS(s)
ZnS(s)
A) 0.560 g
B) 1.12 g
C) 1.50 g
D) 2.24 g
E) 2.28 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q61: How many moles of water are produced
Q62: Which of the following steps is necessary
Q63: How many moles of oxygen gas react
Q64: Assuming similar conditions,how many liters of chlorine
Q65: What is the term for the substance
Q67: What is the mass of aluminum oxide
Q68: How many moles of lithium metal react
Q69: In an experiment,0.197 g of gold metal
Q70: What is the mass of bismuth carbonate
Q71: In general,how many unit factors are required