Multiple Choice
How many molar masses of HCl gas are produced from 1 molar mass (MM) of hydrogen gas according to the following equation? __ H₂(g) + __Cl₂(g) 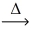 __HCl(g)
__HCl(g)
A) 1 MM
B) 2 MM
C) 3 MM
D) 4 MM
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q127: In general,how many unit factors are required
Q128: What is the term for iron that
Q129: What common household chemical is produced from
Q130: Considering the limiting reactant concept,how many moles
Q131: What is the mass of sodium metal
Q133: In general,how many unit factors are required
Q134: What is the mass of copper metal
Q135: What volume of oxygen gas reacts to
Q136: What is the mass of aluminum metal
Q137: Classify the following type of stoichiometry problem: