Multiple Choice
Using atomic notation,indicate the isotope having 26 p+,32 n0,and 26 e-.
A) 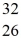 Fe
Fe
B) 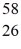 Fe
Fe
C) 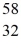 Fe
Fe
D) 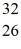 S
S
E) 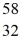 S
S
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q57: In 1895 lines were observed in the
Q58: Given that the only naturally occurring isotope
Q59: How many energy sublevels exist within in
Q60: What is the term for the atomic
Q61: Which of the following represents the quantum
Q63: Which element has the following electron configuration:
Q64: Which of the following gaseous elements produces
Q65: What is the maximum number of electrons
Q66: How many energy sublevels theoretically exist in
Q67: How many photons of light are emitted