Multiple Choice
Using atomic notation,indicate the isotope having 30 p+,35 n0,and 30 e-.
A) 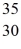 Zn
Zn
B) 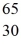 Zn
Zn
C) 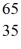 Zn
Zn
D) 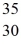 Br
Br
E) 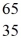 Br
Br
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q83: Which of the following subatomic particles is
Q84: Using atomic notation,indicate the isotope having 11
Q85: Which of the following wavelengths of light
Q86: What is the term for the distance
Q87: What is the maximum number of electrons
Q89: What is the term for the value
Q90: Which of the following energy-level changes for
Q92: Which of the following energy level changes
Q93: What is the term for the spatial
Q98: What is the term for the shorthand