Multiple Choice
If a sample of ammonia gas,NH₃,reacts with oxygen and platinum catalyst to give 55.5 L of nitrogen monoxide gas at STP,what mass of ammonia reacted? NH₃(g) + O₂(g) 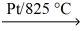 NO(g) + H₂O(g)
NO(g) + H₂O(g)
A) 33.7 g
B) 42.2 g
C) 52.7 g
D) 63.2 g
E) 73.1 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: What principle states that mass is neither
Q16: If 0.600 mol of A reacts with
Q17: What is the term for a chemical
Q18: What is the term that describes the
Q20: If the unknown quantity is g/cm3,what is
Q22: How many water molecules are in a
Q23: After carefully reading a stoichiometry problem,what must
Q24: Given the following two-step process for converting
Q26: Which of the following units is an
Q31: What term expresses the ratio of moles