Essay
Solution Volumes
An analytical chemist wanted to use electrolysis to determine the number of moles of cupric ions in a given volume of solution. The solution was partitioned into n = 30 portions of 0.2 mL each. Each of the n = 30 portions was tested. The average number of moles of cupric ions for the n = 30 portions was found to be 0.185 mole; the standard deviation was 0.015 mole.
-Refer to the Solution Volumes statement. Calculate the intervals ( 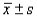 ), (
), ( 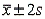 ), and (
), and ( 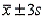 ).
).
Correct Answer:

Verified
(  ) = 0.185
) = 0.185  0.015 or 0.170 t...
0.015 or 0.170 t...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q58: If a distribution is strongly skewed by
Q59: Tchebysheff's Theorem states the following: Given a
Q60: Aptitude Tests<br>Twenty-eight applicants interested in working in
Q61: Ice Cream Cone Sales<br>A neighbourhood ice cream
Q62: Amount of Food Sold<br>Suppose the hourly dollar
Q64: The mode is the sum of a
Q65: Which of the following randomly selected measurements,
Q66: Aptitude Tests<br>Twenty-eight applicants interested in working in
Q67: In assembling a home appliance, workers generally
Q68: A sample of 600 values produced the