Multiple Choice
Consider the following phase diagram of water. The temperature and pressure scales are greatly reduced (and are non-linear) 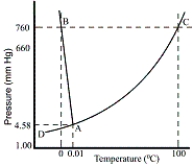
-The substance shown in the image is dry ice (solid CO2) which is composed of molecules in the crystal lattice.  What type of intermolecular force holds the molecules together in the crystal?
What type of intermolecular force holds the molecules together in the crystal?
A) London dispersion forces
B) dipole-dipole interactions
C) hydrogen bonding
D) More than one of the above may be present.
Correct Answer:

Verified
Correct Answer:
Verified
Q54: Which of the following molecules can engage
Q63: In which state of matter are the
Q64: A certain quantity of neon gas is
Q66: Which regions of a heating curve correspond
Q67: Consider the following phase diagram of water.
Q69: Which physical state of matter could be
Q70: Which of the following types of solid
Q71: Which of the following does not affect
Q72: If the change from A to B
Q73: Consider the following phase diagram of water.