Multiple Choice
Consider the following heating curve for the generic substance Z. 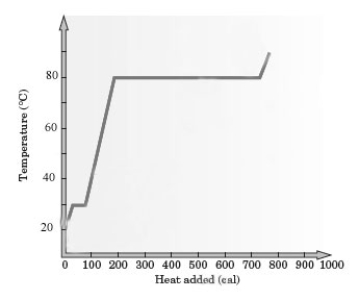
-About how much energy was added to convert all of this sample of Z from the liquid at the boiling point to the gas at the boiling point?
A) 60 cal
B) 80 cal
C) 550 cal
D) 780 cal
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: The normal boiling point of acetic acid
Q40: Which of the following is a mathematical
Q41: At constant temperature the pressure on a
Q42: Which of the following will occur if
Q43: According to the kinetic molecular theory, which
Q44: A vessel under 2.015 atm pressure contains
Q47: Consider the following phase diagram of water.
Q48: Which of the following molecules can have
Q49: A sample of carbon dioxide occupies 22.4
Q50: If 2.00 moles of NO gas occupies