Multiple Choice
Which of the following represents the correct Lewis dot structure for selenium (Se) ?
A) 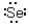
B) 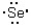
C) 
D) 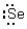
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q220: Lone pairs of electrons do not count
Q221: Which of the following substances is molecular
Q222: Which of the following cations does not
Q223: Carbon may have a +4 or -4
Q224: The number of valence electrons that belong
Q226: Which of the following ions contains oxygen?<br>A)
Q227: Which of the following compounds contains oxygen?<br>A)
Q228: Which of the following AQUEOUS acids does
Q229: In a(n) _ bond, the electrons are
Q230: The compound H₂CO₃ is called _.<br>A) carbonic