Multiple Choice
The compound HA is an acid that is soluble in water. Which of the "beakers" below shows HA behaving as a weak acid in water?
A) 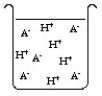
B) 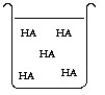
C) 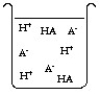
D) Both A and C are weak acids in water.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q36: What were the defining characteristics of bases
Q37: A baking soda solution is a strong
Q38: Which of the following bases is a
Q39: Sodium chloride (NaCl) is considered an electrolyte
Q40: The base in the forward reaction is
Q42: Identify each [H?O+] / [OH-] / pH
Q43: Milk is slightly basic.
Q44: A drop of a full unit in
Q45: The stomach is very sensitive to changes
Q46: Identify each of the substances as an