True/False
The compound HCl is considered a Bronsted-Lowry base because it increases the concentration of 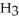
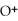 when it dissociates in water.
when it dissociates in water.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q82: Which of the following compounds does not
Q83: Which of the following conjugate pairs would
Q84: Identify each of the acids as a
Q85: Identify each of the substances as an
Q86: Which substance is the most basic?<br>A) blood<br>B)
Q88: Identify each [H?O+] / [OH-] / pH
Q89: What is the pH of an aqueous
Q90: Identify the household products as acidic or
Q91: Solid sodium chloride is a strong electrolyte.
Q92: Identify the household products as acidic or