True/False
The carbonate ( 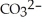 )/bicarbonate (
)/bicarbonate ( 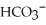 ) pair represents a base and its conjugate acid.
) pair represents a base and its conjugate acid.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: An aqueous solution of pH 9.5 is
Q18: Which of the following acids produces more
Q19: An aqueous solution of HCl is called
Q20: Identify each of the substances as an
Q21: Match each of the acids with the
Q23: Deionized water can conduct electricity.
Q24: The pH of an aqueous solution is
Q25: Identify each of the substances as an
Q26: Identify each of the acids as a
Q27: The conjugate base of ammonia is _.<br>A)