Multiple Choice
Predict the effect of increasing pressure in each of the reactions by choosing the appropriate direction shift.
-CH₄ (g) + 2 O₂ (g) 
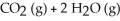
A) no effect
B) right (→)
C) left (←)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q96: The equilibrium constant expression for the reaction
Q97: If the solubility of a certain salt
Q98: Consider the reaction: C (s) + CO?
Q99: Increasing the concentration of hydrogen gas will
Q100: Consider the reaction: C (s) + CO?
Q102: Consider the reaction: C (s) + CO?
Q103: Identify each of the equilibrium constant values
Q104: For this chemical reaction at equilibrium: N₂
Q105: For the following endothermic reaction, what condition
Q106: The solubility product expression for silver(I) sulfate