Multiple Choice
Predict the effect of increasing the concentration of the bolded species in each of the balanced equations by choosing the appropriate direction shift.
-2 SO₃ (g) + 2 CL₂ (g) 
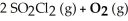
A) left (←)
B) right (→)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q110: Adding more oxygen will _. 2SO₂ (g)
Q111: The equilibrium condition of a chemical reaction
Q112: Adding a catalyst will _. FeCL₃ +
Q113: Equal numbers of moles for A and
Q114: Removing sulfur dioxide as it is formed
Q116: The concentrations of reactants and products do
Q117: Consider the reaction with Kc = 0.211.
Q118: A product favored reaction has Keq =
Q119: Increasing the pressure will _. 2NH₃ (g)
Q120: Write an expression for the equilibrium constant