Essay
Examine this reaction coordinate diagram, and answer the questions that follow. 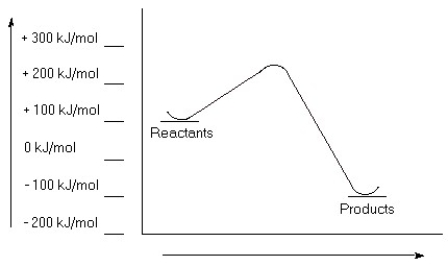 a) Estimate the value for the activation energy for this reaction.
a) Estimate the value for the activation energy for this reaction.
b) Calculate ΔErxn.
c) Is this reaction exothermic or endothermic?
Correct Answer:

Verified
a) 150 kJ/...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q88: If the reaction has a negative overall
Q89: Which of the following represents a reaction
Q90: Match the rate of each reaction with
Q91: Based on the collision theory, decreasing temperature
Q92: The rate determining step is always the
Q94: Consider the reaction X → Y with
Q95: The following data was obtained at 100
Q96: Which of the following is an exothermic
Q97: The order of the reaction with respect
Q98: Which of the following statements regarding reaction