Essay
Write the overall balanced equation that is consistent with the following mechanism:
Step 1: 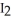 ↔
↔ 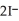 (fast)
(fast)
Step 2: 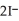 +
+ 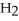 → 2HI (slow)
→ 2HI (slow)
Next, give a rate law expression that is consistent with this proposed mechanism.
Explain a series of three experiments that could help you to determine the values for the overall order of reaction, and the orders of the individual terms in your proposed rate law expression.
Correct Answer:

Verified
I₂ + H₂ → 2HI
rate = k[H₂][I₂]
Any varia...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
rate = k[H₂][I₂]
Any varia...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q6: If the kinetic energy of the reactant
Q7: Compare the roles of catalysts and enzymes
Q8: In each of the following diagrams, the
Q10: Examine this reaction coordinate diagram and answer
Q12: Based on the collision theory, adding a
Q13: In an exothermic reaction, more energy is
Q14: For chemical reactions to occur at all,
Q15: The transition state for a reaction occurs
Q16: The above data were obtained in a
Q41: Match the rate of each reaction with