Multiple Choice
Using retrosynthetic synthesis, determine which compound(s) could lead to the alcohol shown below in a single step: 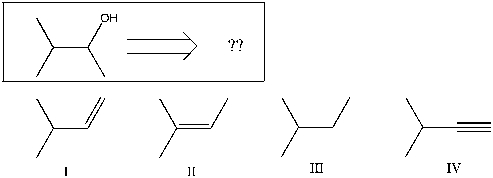
A) I
B) II
C) III
D) IV
E) I or II
) I, II, or IV
Correct Answer:

Verified
Correct Answer:
Verified
Q37: Starting with a primary alkyl bromide, which
Q74: Using retrosynthetic synthesis, determine which compound(s) could
Q75: Propose an efficient method of converting 3-methyl-1-butanol
Q76: Propose an efficient method of completing the
Q77: 2-Methylpentane (C<sub>6</sub>H<sub>14</sub>) can be converted, through a
Q78: Predict the products of the following reaction:
Q80: For the transformation shown, select the most
Q81: Propose an efficient synthesis of cyclopentanone from
Q82: Devise an efficient synthesis of the diol
Q83: Select the best reagent to convert 4,5-dimethylhex-2-yne