Multiple Choice
Consider the following SN2 reaction, 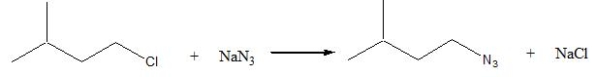 Assuming no other changes, what is the effect on the rate, if the concentration of both 1-chloro-3-methylbutane and NaN3 is doubled?
Assuming no other changes, what is the effect on the rate, if the concentration of both 1-chloro-3-methylbutane and NaN3 is doubled?
A) No effect
B) It would double the rate
C) It would triple the rate
D) It would increase four times
E) It would reduce by half
Correct Answer:

Verified
Correct Answer:
Verified
Q113: What is the IUPAC name for the
Q114: Provide a curved arrow mechanism for the
Q115: Predict the major product(s) and provide a
Q116: Provide the reagents necessary to carry out
Q117: Which of the following compounds will undergo
Q118: Provide the reagents necessary to carry out
Q119: Provide a curved arrow mechanism for the
Q120: Provide the reagents necessary to carry out
Q123: What is the correct structure for 3-ethyl-1-iodocyclohexane?
Q213: Which of the following describes the difference