Multiple Choice
The following compound is best classified as a ____. 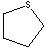
A) Brønsted-Lowry acid
B) Lewis acid
C) Brønsted-Lowry base
D) Lewis base
E) Both C & D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: Which of the following compounds is most
Q5: Which of the indicated protons is most
Q6: Determine if NaOH is a suitable reagent
Q8: For the following reaction, which reactant functions
Q10: Which is the conjugate acid in the
Q11: A Brønsted-Lowry acid is defined as a
Q12: A Lewis base is defined as _.<br>A)
Q14: Identify the acid and the base and
Q29: CH<sub>3</sub>CH<sub>2</sub>OCH<sub>2</sub>CH<sub>3,</sub> is best classified as a _.<br>A)
Q71: Which of the following compounds is most