Multiple Choice
The lone pair on nitrogen in the following compound is _______. 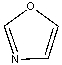
A) localized
B) delocalized
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: How many lone pairs of electrons are
Q34: How many hydrogen atoms are connected to
Q35: What is the formal charge on the
Q36: Which of the following compounds have +1
Q39: Draw the resonance structure indicated by the
Q40: How many hydrogen atoms are connected to
Q41: The indicated bond in the following compound
Q42: Provide correct condensed structure for the following
Q43: How many hydrogen atoms are connected to
Q161: Draw the Lewis structure for (CH<sub>3</sub>)<sub>3</sub>C(CH<sub>2</sub>)<sub>2</sub>OCH(CH<sub>2</sub>CH<sub>3</sub>)<sub>2</sub>.