Multiple Choice
Consider the reaction of A being converted into B at 25°C. If the ΔG° of this reaction is 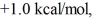 the Keq is ________ and the % conversion is ________.
the Keq is ________ and the % conversion is ________.
A) 0.18, 15%
B) 0.43, 30%
C) 1.0, 50%
D) 2.3, 70%
E) 5.4, 84%
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q28: Which of the following is not a
Q31: Given the bond dissociation energies below (in
Q44: What reactive intermediate is formed when CHBr<sub>3
Q54: Which of the following is a propagation
Q78: Consider the reaction: CH<sub>3</sub>CH<sub>2</sub>∙ + Br<sub>2</sub> →
Q79: Within the visible spectrum, it has been
Q83: Given a K of 2.2 at 25°C,
Q84: For the reaction A + B →
Q92: Use the Hammond Postulate to explain why
Q115: Energy is _ when bonds are formed