Multiple Choice
Which of the presented mechanisms would be the most energetically favorable and thus the most likely mechanism to actually occur for the following free radical chain reaction? (bond dissociation energies -- H-H = 104 kcal/mol; Cl-Cl = 58 kcal/mol; H-Cl = 103 kcal/mol)
H2 + Cl2
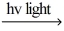 2 HCl
2 HCl
A) H2 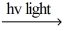 H∙ + H∙
H∙ + H∙
H∙ + Cl2 → Cl∙ + HCl
H∙ + Cl∙ → HCl
B) Cl2 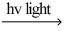 Cl∙ + Cl∙
Cl∙ + Cl∙
Cl∙ + H2 → H∙ + HCl
H∙ + Cl2 → HCl + Cl∙
C) H2 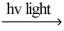 H∙ + H∙
H∙ + H∙
H∙ + Cl2 → Cl∙ + HCl
Cl∙ + H2 → HCl + H∙
D) Cl2 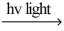 Cl∙ + Cl∙
Cl∙ + Cl∙
Cl∙ + H2 → H∙ + HCl
H∙ + Cl∙ → HCl
Correct Answer:

Verified
Correct Answer:
Verified
Q6: The Arrhenius equation mathematically models which of
Q11: The bond dissociation energy is the amount
Q55: When acetaldehyde (CH<sub>3</sub>CHO) is deprotonated, the resulting
Q56: How many distinct monochlorinated products can result
Q91: What reactive species is produced in the
Q103: What is the name of the major
Q112: Explain the significance of the exponential factor
Q117: Consider the one-step conversion of F to
Q119: Which of the following is a carbene?<br>A)
Q121: Which of the following depictions most closely