Essay
Consider the conversion of X to Z through the sole intermediate Y. Given the reaction-energy diagram shown below, which step is the rate-limiting step? Explain your reasoning. 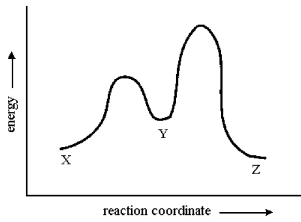
Correct Answer:

Verified
The conversion of Y to Z has a...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q6: The Arrhenius equation mathematically models which of
Q9: When two carbenes collide, they immediately dimerize
Q21: What is the relative reactivity of 2°
Q50: In the reaction of Cl<sub>2</sub> with ethane
Q85: Consider the elementary step in the solvolysis
Q104: Which of the following reactive intermediate species
Q107: Predict the enthalpy (ΔH) value for the
Q110: Provide the major organic product(s) in the
Q112: Explain the significance of the exponential factor
Q117: Consider the one-step conversion of F to