Essay
Starting with cyclohexene and employing a Dieckmann cyclization show how the compound below can be prepared. 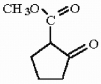
Correct Answer:

Verified
1. KMnO4, -OH, Δ
2. H+
...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
2. H+
...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q3: Disregarding stereoisomers, how many different enols can
Q7: What crossed-aldol product results when butanal is
Q12: When compound X is heated, PhCOCH(CH<sub>3</sub>)<sub>2</sub> and
Q28: The Hell-Volhard-Zelinsky reaction involves _.<br>A) the α-bromination
Q57: Provide a detailed, stepwise mechanism for the
Q62: Provide the major organic product of the
Q63: Provide the sequence of synthetic steps necessary
Q64: Provide the structure of the aldol product
Q68: Complete the following short synthesis by providing
Q70: What product results when malonic ester is