Short Answer
What kind of molecular orbital (σ, σ*, π, or π*) results when the two atomic orbitals shown below interact in the manner indicated? 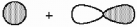
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q59: What is the approximate value of any
Q65: Which functional groups below indicate the presence
Q79: Choose the correct hybridization for the atom
Q82: What kind on molecular orbital (σ, σ<sup>*</sup>,
Q83: How many π bonds are present in
Q87: Are the two compounds shown below
Q88: Circle the coplanar atoms in 1-ethylcyclopentene shown
Q89: The HCC bond angle in allene (H<sub>2</sub>C=C=CH<sub>2</sub>)
Q95: Would you expect sodium chloride (NaCl)to be
Q95: Use the following structure for the two