Multiple Choice
Which of the following statements concerning the cyclic molecule shown is not true? 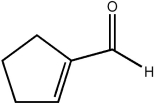
A) It contains a π molecular orbital formed by the overlap of a carbon p atomic orbital with an oxygen p atomic orbital.
B) It contains a σ molecular orbital formed by the overlap of two carbon sp2 hybrid atomic orbitals.
C) It contains a σ molecular orbital formed by the overlap of two carbon sp3 hybrid atomic orbitals.
D) It contains a π molecular orbital formed by the overlap of two carbon p atomic orbitals.
E) It contains a σ molecular orbital formed by the overlap of a carbon p atomic orbital with an oxygen sp3 atomic orbital.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Which molecule below is an alkene?<br>A) CH<sub>3</sub>CH<sub>2</sub>OCH<sub>2</sub>CH<sub>3</sub><br>B)
Q22: Which of the molecules below has the
Q90: Which of the molecules below can hydrogen
Q105: Choose the correct hybridization for the atom
Q119: The CCO bond angle in acetone (CH<sub>3</sub>COCH<sub>3</sub>)
Q119: The HCH bond angle in propane (CH<sub>3</sub>CH<sub>2</sub>CH<sub>3</sub>)
Q120: Vildagliptin is a recently released antidiabetic
Q121: How many s bonds does the compound
Q122: The synthetic steroid RU-486 is shown below.
Q123: The compounds below are base pairs used