Short Answer
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 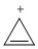
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: Classify the compound below as aromatic, antiaromatic,
Q42: In the carbon NMR, in what region
Q43: Provide an acceptable name for the compound
Q45: Which of the labeled H atoms (1
Q48: Which sequence ranks the indicated protons in
Q50: Describe the occupied p molecular orbitals in
Q103: Aromatic molecules contain _ π electrons.<br>A) no<br>B)
Q110: Classify cyclopentadienyl cation as aromatic, antiaromatic, or
Q124: Provide an acceptable name for PhSO<sub>3</sub>H.
Q126: Provide the structure of tropylium bromide.